What Is An Example Of A Buffer In Biology
For example a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a pH of about 925. TBS has a neutral pH and is often used as a wash or dilution buffer in biological assays.
 Western Blot Structural Biology Gel Algebra Formulas
Western Blot Structural Biology Gel Algebra Formulas
If too much H enters the body bicarbonate will combine with the H to create carbonic acid and limit the decrease in pH.

What is an example of a buffer in biology. In this system the weak acid dissociates to a small extent giving bicarbonate ions. Two common buffer examples include tris hydroxymethylaminomethane tris-buffered saline TBS and 4- 2-hydroxyethyl-1-piperazineethanesulfonic acid HEPES. Alkaline Basic buffer solution.
For example the bicarbonate buffering system is used to regulate the pH of blood. These are solutions that have a pH above 7 and contain a weak base and one of its salts. They play a major role in the anatomy of every human being.
Since pH is a measure of hydrogen ion concentration the presence of a buffer keeps the pH of a solution constant within a narrow range. For example a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a pH of about 925. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.
Buffer solutions help in the adjustment of the nature of blood. An example of the use of buffers in pH regulation is the use of bicarbonate and carbonic acid buffer system in order to regulate the pH of animal blood. For example if you put the strong base sodium hydroxide NaOH in water it becomes Na.
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. What is neutralization a reaction. These are solutions that have a pH below 7 and contain a weak acid and one of its salts.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Buffer solutions are also used to maintain an optimum pH for enzyme activity in many organisms. For example a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 475.
For example a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 475. For example one buffer may effectively buffer a solution between pH 6 and 65 while another may function well between pH 8 and 83. Weak AcidBases and Buffers When acids and bases are strong it just means they completely ionize in water.
Extraction buffers also sometimes referred to as the lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the compounds of the cells. Buffers typically consist of an acid-base pair with the acid and base differing by the presence or absence of a proton a conjugate acid-base pair. In this buffer hydronium and bicarbonate anion are in equilibrium with carbonic acid.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
Blood as a Buffer Solution. Most buffers consist of a weak acid and a weak base. Also glucose is a monomer of a carb.
In nature there are many systems that use buffering for pH regulation. HEPES is a zwitterionic buffer that is commonly used in cell culture. An example would be 1 train cart and many of those train carts make up the train which is the polymer.
This buffer system involves carbonic acid H 2 CO 3 and bicarbonate HCO 3 anion. If the alkaline nature of blood increases buffer solutions tend to bring down the pH value of blood. Similarly adding water to a buffer or allowing water to evaporate will not change the pH of a buffer.
It keeps the pH within the proper range. Not all buffers operate in the same range. For instance one of the buffers that maintain the pH of human blood involves carbonic acid H _2 2.
If you add an acid or a base to a buffered solution its pH will not change significantly. Alkaline buffers on the other hand have a pH above 7 and contain a weak base and one of its salts. Most lysis buffers contain salts to regulate the acidity and osmolarity of the lysate.
If acid is added to this buffer the added H ions combine with bicarbonate ions to produce more carbonic acid using up some of the H ions the Na ions do not participate in this reaction. Blood itself tends to be a buffer solution by keeping its pH value constant. Blood Human blood contains a buffer of carbonic acid H 2 CO 3 and bicarbonate anion HCO 3- in order to maintain blood pH between 735 and 745 as a value higher than 78 or lower than 68 can lead to death.
Learn vocabulary terms and more with flashcards games and other study tools. For example blood contains a carbonic acid H 2 CO 3-bicarbonate HCO 3- buffer system. Carbon dioxide is part of a prominent buffer system in the human body.
Its pH changes very little when a small amount of strong acid or base is added to it. What is a buffer. Learn buffer biology with free interactive flashcards.
Choose from 498 different sets of buffer biology flashcards on Quizlet. A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3. Start studying Biology chapter 2 and 3.
They help maintain a given pH even after the addition of an acid or a base.
 Ap Biology Homeostasis And Feedback Review Ap Biology Biology Resources Biology
Ap Biology Homeostasis And Feedback Review Ap Biology Biology Resources Biology
 Genetic Testing Hopes Biology Labs Science Biology Biology Classroom
Genetic Testing Hopes Biology Labs Science Biology Biology Classroom
 Buffer Capacity Buffers Titrations And Solubility Equilibria Chemi Solubility Chemistry Ap Chemistry
Buffer Capacity Buffers Titrations And Solubility Equilibria Chemi Solubility Chemistry Ap Chemistry
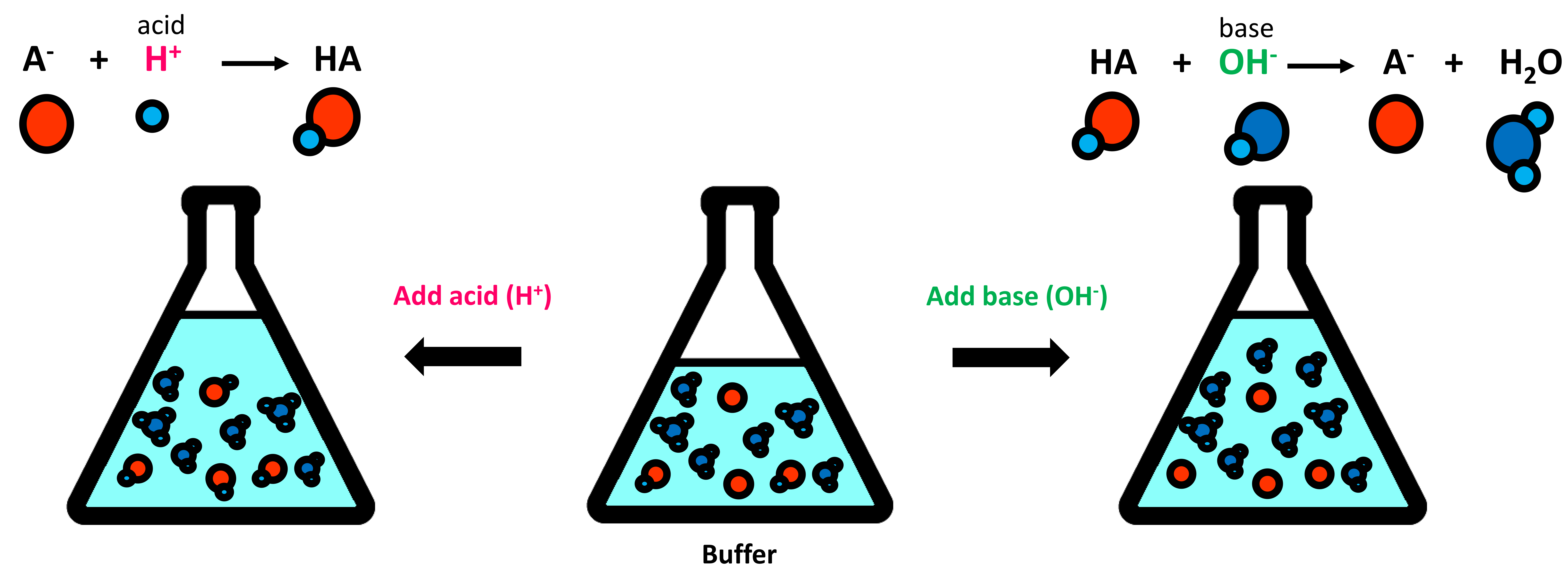 What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
 Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtub Teaching Chemistry Science Chemistry Biochemistry Notes
Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtub Teaching Chemistry Science Chemistry Biochemistry Notes
 Pin By Bnb On 2d Art Teaching Chemistry Chemistry Lessons Apologia Chemistry
Pin By Bnb On 2d Art Teaching Chemistry Chemistry Lessons Apologia Chemistry
 The Body S Buffer System And Ph Imbalances Helpful Youtube Video Nursing School Notes Respiratory Therapy Medical Laboratory Scientist
The Body S Buffer System And Ph Imbalances Helpful Youtube Video Nursing School Notes Respiratory Therapy Medical Laboratory Scientist
 Buffers Definition Overview Expii
Buffers Definition Overview Expii
 Acids Bases Draw It To Know It Study Chemistry Chemistry Education Biochemistry Notes
Acids Bases Draw It To Know It Study Chemistry Chemistry Education Biochemistry Notes
 Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
 Differential Centrifugation Biology Units Photosynthesis Organelles
Differential Centrifugation Biology Units Photosynthesis Organelles







